Perry County Health Department supports the health and well being of its residents.
Contact Perry County Health Dept.
Perry County Health Department
31 Medical Drive
Linden, TN 37096-3326
(931) 589-2138 (Main)
(931) 589-5414 (Domestic Fax)
Regional Office
South Central Regional Office:
1216 Trotwood Ave.
Columbia, TN 38401-6406
(931) 380-2532
(931) 380-3364 Fax
Perry County Health Department is now offering vaccine to the 1ab group, 12+ years and older. In the meantime everyone should wear a mask to prevent the further spread of COVID-19.
Perry County Health Department
31 Medical Drive
Linden, TN 37096-3326
(931) 589-2138 (Main)
(931) 589-5414 (Domestic Fax)
SERVICES OFFERED BY PERRY COUNTY HEALTH DEPARTMENT
Perry County
Under the guidelines of the State of Tennessee Health Department, the Perry County Health Department currently is serving the Moderna vaccine for age 12+ and older.
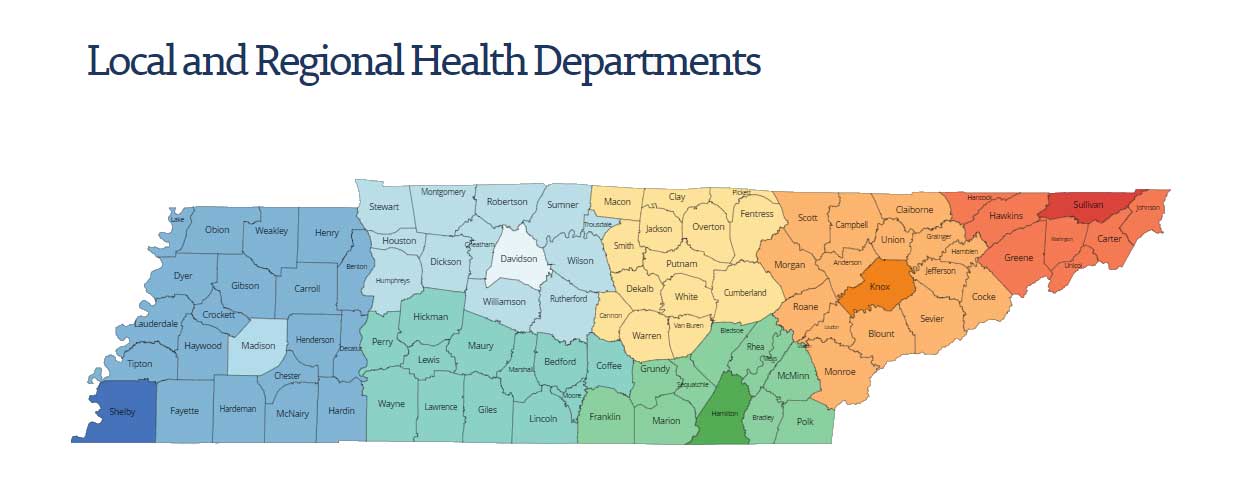
Each county in Tennessee has a county health department, with some larger counties having multiple facilities. A total of 89 primarily rural county health departments operate under the direct supervision of the Tennessee Department of Health, headquartered in Nashville, while the six larger, urban counties – Madison, Shelby, Knox, Davidson, Hamilton and Sullivan – have health departments that operate under local governance but work closely with the Tennessee Department of Health.
Directors of the 89 primarily rural health departments are appointed by the Commissioner of the Tennessee Department of Health. Directors of the six larger, urban county health departments are appointed by their county leadership. In some instances, a health board appointed by the mayor will provide recommendations regarding these appointments. Here are links to the larger, urban county health departments in Tennessee:
Services provided by rural and urban health departments vary, reflecting population health needs and the capacity of funding available from year to year.
COVID-19 Vaccine Information
How to get a Vaccine
How to Get a COVID-19 Vaccine
There are many COVID-19 vaccines currently in development and in clinical trials. The availability of a safe and effective COVID-19 vaccine depends on the outcomes of the Phase 3 clinical trials that are currently underway. If and when a vaccine is shown to be safe and effective in the Phase 3 clinical trials, it will be issued authorization by the Food and Drug Administration (FDA) for use in a limited number of individuals who are at high-risk for developing COVID-19 or who are at high risk of serious complications from COVID-19. The vaccine will continue to be monitored as it is used in larger populations to ensure that any indication of rare complications from its use are detected as soon as possible and evaluated to see if they were caused by the vaccine.
The Tennessee Department of Health (TDH) is working to ensure that a vaccine can be allocated and distributed to priority populations once that is made official. TDH will continue to monitor and share information about the COVID-19 vaccine on this webpage as it becomes available.
Vaccination Communication Toolkit
General COVID-19 Vaccine Information
Tennessee COVID-19 Vaccine Allocation and Distribution Plan
COVID-19 Vaccine Specific Information





